




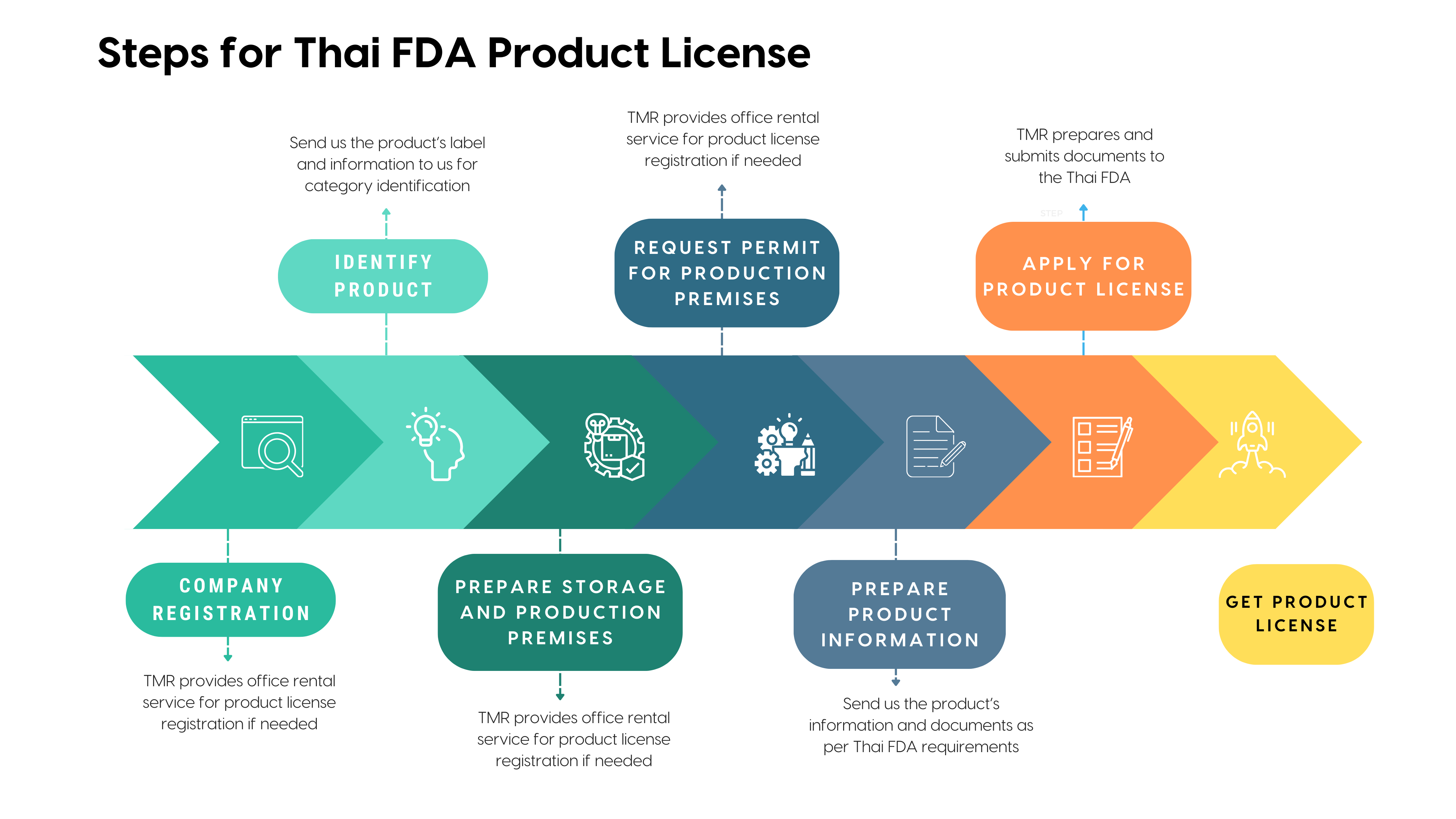
Preparing the premises for importing or ordering food supplements into the Kingdom of Thailand can be divided into 2 parts:
1. Food supplements Importation Premises (Office)
2. Food supplements Storage Premises
NOTE: Navigating FDA product license registration without a local company? We provide comprehensive support for seamless license submission, ensuring compliance every step of the way. Connect with us for hasslefree assistance in meeting regulatory requirements, allowing you to handle the importation or ordering of Food supplements into the Kingdom of Thailand seamlessly
Categorizing the products before submitting the registration application can help ensure accurate submission and reduce the processing time.
1. Application form
2. Copy of the company's certification
3. Copy of the company's registration
4. Copy of the house registration of the production location
5. Copy of the house registration and ID card of the directors
6. Photographs of the import location and equipment
7. Location map and coordinates of the production location
8. Various certifications
9. Medical certification
1. Name and formula of 100% production
2. Production procedure
3. Consumption method/ consumption quantity
4. Product label
5. Test results (if any)
6. Specifications
7. Certificate of analysis
8. Details of packaging
9. Storage method / storage life
10. Certificate of production / import location
11. Quality specifications for important components
12. Documents certifying quality standards (ISO 22000, HACCP and FSSC)
13. Application form and other documents as required by the food group.
Preparing the premises for importing or ordering cosmetic into the Kingdom of Thailand can be divided into 2 parts:
1. Cosmetic Importation Premises (Office)
2. Cosmetic Storage Premise
NOTE: Navigating FDA product license registration without a local company? We provide comprehensive support for seamless license submission, ensuring compliance every step of the way. Connect with us for hasslefree assistance in meeting regulatory requirements, allowing you to handle the importation or ordering of cosmetic into the Kingdom of Thailand seamlessly
The process of applying for product license for each type of product may vary, and it must be submitted correctly according to the type of product.
1. Application form
2. Copy of the company's certification
3. Copy of the company's registration
4. Copy of the house registration of the production location
5. Copy of the house registration and ID card of the directors
6. Photographs of the import location and equipment
7. Location map and coordinates of the production location
8. Various certifications
9. Medical certification
1. Name and formula of 100% production
2. Production procedure
3. Consumption method/ consumption quantity
4. Product label
5. Test results (if any)
6. Specifications
7. Instruction for use
8. Storage life
9. Application documents and other documents as specified by the Cosmetic Control Group
Preparing the premises for importing or ordering medical device into the Kingdom of Thailand can be divided into 2 parts:
1. Medical device Importation Premises (Office)
2. Medical device Storage Premises
NOTE: Navigating FDA product license registration without a local company? We provide comprehensive support for seamless license submission, ensuring compliance every step of the way. Connect with us for hasslefree assistance in meeting regulatory requirements, allowing you to handle the importation or ordering of medical device into the Kingdom of Thailand seamlessly
The process of applying for product license for each type of product may vary, and it must be submitted correctly according to the type of product.
1. Application form
2. Copy of the company's certification
3. Copy of the company's registration
4. Copy of the house registration of the production location
5. Copy of the house registration and ID card of the directors
6. Photographs of the import location and equipment
7. Location map and coordinates of the production location
8. Various certifications
9. Medical certification
1. Instruction for use
2. Product label
3. User manual
4. Documents certifying quality standards (ISO 13485)
5. Production and Material Flow chart
6. Application documents and other documents as specified by the Medical Device Control Division
7. Certificate of Free Sales (CFS)
Preparing the premises for importing or ordering drug into the Kingdom of Thailand can be divided into 2 parts:
1. Drug Importation Premises (Office)
2. Drug device Storage Premises
NOTE: Navigating FDA product license registration without a local company? We provide comprehensive support for seamless license submission, ensuring compliance every step of the way. Connect with us for hasslefree assistance in meeting regulatory requirements, allowing you to handle the importation or ordering of drug into the Kingdom of Thailand seamlessly
The process of applying for product license for each type of product may vary, and it must be submitted correctly according to the type of product.
1. Application form
2. Copy of the company's certification
3. Copy of the company's registration
4. Copy of the house registration of the production location
5. Copy of the house registration and ID card of the directors
6. Photographs of the import location and equipment
7. Location map and coordinates of the production location
8. Various certifications
9. Medical certification
1. Primary & Secondary Packaging Labels
2. Drug Master File (DMF)
3. Thai & English Product Labeling
4. Sample Product for Analysis purpose (If any)
5. Good Manufacturing Practice (GMP) Certificate
6. Factory Process Assessment Reports
7. Rectification Plan
8. Site Master File
9. Quality Manual
10. Certificate of Pharmaceutical Product (CPP)
11. Application documents and other documents as specified by the Thai FDA


